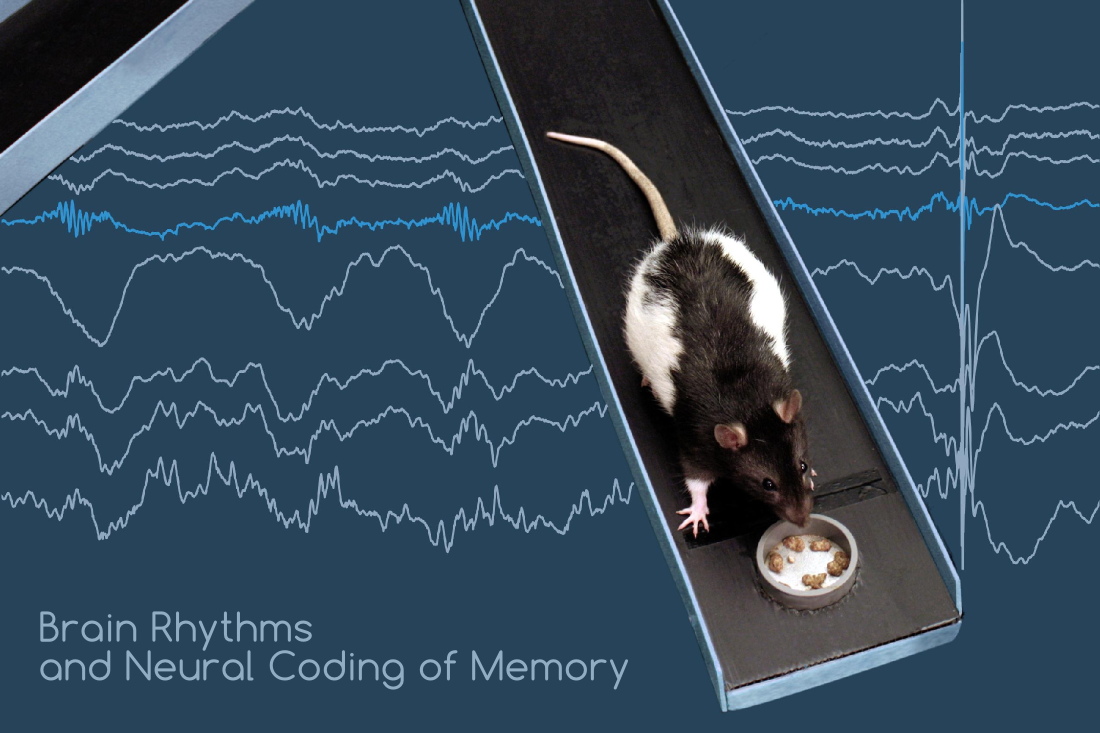
The hippocampus is a limbic structure that plays a critical role in the formation, consolidation and recall of various forms of memory, including episodic and spatial memory. How do such complex cognitive functions emerge from the activity of hippocampal neurons? One key property of the hippocampal network that has gained tremendous interest in recent years is its ability to encode and reactivate sequential activity patterns: specific subgroups of neurons become successively active, either in response to the ongoing behavioral and cognitive context, or spontaneously during recall, decision making and sleep. In the spatial domain, fast sequential activation of hippocampal ‘place cells’ anticipates future trajectories, associates traveled paths with rewarding or aversive stimuli, and appears to underlie certain forms of spatial learning. During subsequent sleep, reactivation of the same sequences plays a critical role in memory consolidation via a hippocampo-cortical dialogue. This versatile capacity to manipulate and memorize abstract information in the form of neural sequences may provide a unifying conceptual framework to reconcile the numerous functions of the hippocampus.
The goal of our research is to decipher the various roles of hippocampal dynamics, including their interactions with cortical and subcortical activity (in e.g. prefrontal cortex, medial and lateral entorhinal cortices, ventral and dorsal striatum, locus cœruleus, ventral tegmental area, etc.), and dissect their network mechanisms in freely moving rodents. We combine several cutting-edge technologies, including massively parallel electrophysiology across multiple brain areas, an innovative optical imaging approach, and optogenetics. We perform state-of-the-art electrophysiological recordings and are developing optical imaging and targeting approaches for real-time optical excitation or inhibition of single, identified neurons, as well as long-term monitoring of vast neural populations — all in freely moving rodents performing complex behavioral tasks in large scale mazes and sleeping. Advanced analytical and statistical methods allow us to unravel the mechanisms and roles of hippocampal sequences in the formation, consolidation and recall of memory.
Our team includes researchers with backgrounds in neurophysiology, animal behavior, physics and engineering. We are part of the Center for Interdisciplinary Research in Biology at the Collège de France, located in the historical center of Paris.
Selected Publications








Other Publications






















The neurobiological basis of memory formation and retrieval has been the major focus of my research over the past four decades. Combining in vivo electrophysiology and pharmacology with astute behavioral analysis, my thesis and subsequent publications provided early challenges the consolidation hypothesis. We published the first paper demonstrating ‘reconsolidation after reactivation of memory’ and showed the importance of the noradrenergic system in this reconsolidation processs. We study the role of the noradrenergic locus coeruleus (LC) in cognitive processes by recording the activity of neurons in this nucleus in behaving rats, engaged in various cognitive tasks. In this way we have elucidated the functional role of this tiny nucleus in modulating encoding and off-line memory consolidation, in concert with activity in frontal cortex and hippocampus. More recently, we have been investigating the role of sleep oscillations and associated LC activity in modulation of memory processes. Currently we are using optogenetic methods to stimulate or inhibit activity of LC neurons at critical periods during learning or during off-line memory consolidation.
Born and educated in New York City, Susan J. Sara received a BA in psychology from Sarah Lawrence College and a PhD from University of Louvain (Belgium). After post doctoral studies at Oxford University and NYU Medical School, she was recruited into the CNRS (France). She founded the group ‘Neuromodulation and Cognitive Processes’ at the University Paris VI, which she headed until her retirement. She is currently Directrice de Recherche Emerite at the College de France and Adjunct Professor at New York University Medical School. SJS was a visiting Professor at the Institute of Neurosciences in Shanghai and at Yamaguchi Medical School, Japan and a visiting scientist at the Weizmann Institute, Israel. She is past president of the European Brain and Behavior Society and has served as Chair of FENS_IBRO schools committee, Chair of IBRO Alumni committee, FENS programme committee, FENS executive Committee. She has served on the Scientific Advisory Board of the Nencki Institute (Poland) and the Max Planck Insitute for Biological Cybernetics (Tuebingen), as well as on several advisory panels for the EU and ERC. She is past editor of Neural Plasticity and currently associate editor of Frontiers in Behavioral Neuroscience.
Book Chapters

We use state-of-the art technologies. Our 256 channel recording systems, multisite silicon probes and custom designed hexatrode/octrode microdrives allow us to simultaneously record from dozens of neurons across multiple brain areas, such as hippocampal place cells and prefrontal cell assemblies. To train animals in spatial learning tasks, we use custom automated setups ranging from standard T-mazes to miniature treadmills transported by model trains. Our data processing infrastructure includes a 96-core computer cluster, three GPU computing servers, and 6 centralized storage servers. To process, visualize and analyze our data, we use our custom application suite NeuroSuite and Matlab toolbox FMAToolbox.

Team Leader

Researcher

Research Director

Professor NYU

Electronics Engineer

Postdoc

PhD Student

Postdoc

M2 Student

Postdoc

M2 Student

PhD Student

PhD Student

PhD Student

Césure
2024

Postdoc
2017-2022

PhD-Postdoc
2012-2021

M2 Student
2018-2019

M2 Student
2017

M2 Student
2016

Postdoc
2006-2009

M2 Student
2024

M2 Student
2022

PhD-Postdoc
2017-2021

Engineer Student
2018

Invited Researcher
2015-2017

PhD-Postdoc
2006-2014

PhD Student
2005-2009

Engineer Student
2023

M2 Student
2022

PhD Student
2017-2020

PhD-Postdoc
2012-2018

Postdoc
2012-2016

M2 Student
2013

PhD Student
2003-2007

M2 Student
2023

Césure
2021-2022

M2 Student
2020

M2 Student
2018

PhD Student
2011-2016

PhD-Postdoc
2006-2012

Postdoc
2004-2005

M2 Student
2023

Postdoc
2013-2021

PhD Student
2016-2020

M2 Student
2017

M1 Student
2015-2016

M2 Student
2012

PhD Student
2022-2023

Postdoc
2013-2021

M2 Student
2018-2019

PhD Student
2013-2017

M2 Student
2016

Postdoc
2009-2012








It is clearly visible on the pavement of rue Soufflot, in front of the Panthéon – just a few hundred meters from our lab.
Postdoctoral fellows are always welcome to join the lab. Although we cannot currently offer funding, we would be happy to support application to independent grants. The candidate should have a strong background in recordings of large neuronal ensembles and optogenetics in freely behaving rodents. Good Matlab programming skills are welcome. Applicants should send their CV, a cover letter detailing their research experience and interests, and two letters of reference.
PhD grants are awarded by the PSL Doctoral School in July. Typical candidates are M2 students who have performed their research internship in the lab, and have been trained and prepared for their PhD project. However, candidates who can apply for independent funding are welcome to contact us.
Students are welcome to contact us for research internships.

